Abstract
Bromodomain-containing protein 9 (BRD9) functions as a key component of the chromatin remodeling complex in regulation of gene transcription, which is widely implicated in tumorigenicity. Although venetoclax has transformed the paradigm of CLL treatment, some patients are still unable to achieve or maintain clinical remission, indicating the medical need of novel therapeutic target or combinatory regimen. Hence, this study was performed to explore the regulatory mechanism and potential clinical utility of BRD9 in CLL.
At first, aberrantly elevated BRD9 expression was frequently observed in CLL patients (n=94) than healthy donors (n=17) both in mRNA and protein level (donors vs. CLL patients, 0.94 ± 0.41 vs. 10.96 ± 2.01, p=0.03). Furthermore, BRD9 overexpression was identified as the independent prognostic factor in CLL (HR=3.55, p<0.001). The exceptionally upregulation of BRD9 was intensely correlated with inferior prognosis in CLL patients, including overall survival (HR=3.246, p=0.02) and time to first treatment (HR=2.326, p=0.005).
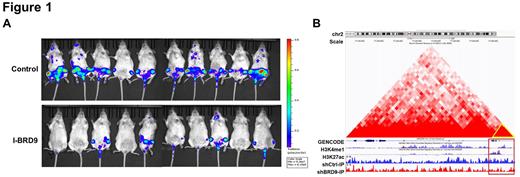
To investigate the functional significance of BRD9 in CLL, stable BRD9 knockdown cells using lentiviral shRNAs were established. The suppression of BRD9 remarkably impeded cell proliferation, increased cell apoptosis and induced G1/S phase blockage in CLL. What's more, I-BRD9, a selective inhibitor of BRD9, resulted in a defective proliferation in CLL primary cells in a time- and dose-dependent manner. We subsequently performed pre-clinical investigations of I-BRD9 in orthotopic CLL xenograft murine model. Notably, I-BRD9 treatment showed obviously reduced leukemia burden and manifested splenomegaly to a lesser extent than control (Figure 1A), which was further confirmed by H&E and Ki67 staining of spleen. Additionally, I-BRD9 could significantly prolong the lifespan (p=0.02) and delay disease progression of the CLL xenograft mice (p<0.01). Also, a reduction of CLL cells in bone marrow and spleen drawn from I-BRD9 treated mice was corroborated by using flow cytometry.
To decipher the specific contribution of BRD9 in CLL pathogenesis, ATAC-seq and CHIP-seq were employed to monitor BRD9-deficent and control cells. Knockdown of BRD9 diminished global ChIP signal and chromatin accessibility near transcriptional start sites. Integrated analysis of mRNA expression profiles of RNA-seq and differential signals of CHIP-seq, Nrf2 was selected as a candidate target for further investigation. The expression of Nrf2 and downstream targets (like HO-1, NOQ1 and SOD1) were significantly decreased in BRD9-silencing CLL cells (p<0.01). Furthermore, the existence of BRD9 binding site was identified in a 2kb region upstream of the NFE2L2 (encoding Nrf2) promoter through constructing truncations (p<0.05). In addition, the enrichment of H3K27ac modification in the binding site was further substantiated by CHIP-qPCR (p<0.01). The inhibitor of histone acetyltransferase reduced the enrichment of BRD9 in this region, indicating that BRD9 bound to NFE2L2 through recognizing the acetylated histone marks. What's more, the Hi-C data suggested a looping of the transcription factor complexes among the labeled regions which showed divergent CHIP signals between shControl and shBRD9 (Figure 1B).
The GO analysis of differentially expressed genes obtained from RNA-seq demonstrated a mainly enrichment in apoptotic pathway, which prompted us to investigate the role of BRD9 in venetoclax sensitivity. Following venetoclax addition, CLL cells with BRD9 silenced presented markedly reduced viability than control cells, indicating BRD9 inhibition could potentially drive a therapeutic vulnerability to the venetoclax (p<0.05). Besides, supplementing I-BRD9 with venetoclax strikingly alleviated malignant proliferation and enhanced apoptosis on CLL primary cells than single agent (all combination index<0.6, p<0.05). Western blotting assays suggested the involvement of Nrf2/ARE signaling in venetoclax sensitivity induced by BRD9 inhibition.
Taking all these data together, our study firstly credentials the oncogenic role of BRD9 in CLL leukemogenesis, suggesting the therapeutic synergy of combinatory venetoclax/I-BRD9 treatment for clinical utility. Targeting BRD9 displays promising clinical application in the context of extensive use of venetoclax in CLL patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.